Artificial Intelligence (AI) is revolutionizing life science sectors such as clinical trials and regulatory compliance. AI speeds up drug development through improved trial design, automation of compliance processes, data management, and streamlining.
1. AI‑Driven Clinical Trial Design and Execution
The Optimal Design of A Trial:
AI can be used to create a paradigm shift in the design of clinical trial protocols. This is done by using large datasets such as historical trial data, real-world evidence, and current patient information. Advanced algorithms simulate scenarios for trials to identify risks, and suggest protocol changes that increase statistical power while reducing unnecessary complexity.
Enhanced Patient Recruitment:
Machine learning models sort through electronic health records and genomic profiles to find and match participants. This can reduce screening time by up to 40% while improving enrollment rates.
Real‑Time Monitoring:
AI-powered pharmacovigilance tools continuously track trial data to detect anomalies and adverse events early. This allows prompt intervention, protecting patient safety.
Together, these AI abilities transform the design and implementation of clinical trials into a dynamic data-centric process. The result is faster, safer, and more efficient drug development–delivering innovative therapies to patients sooner while maintaining high regulatory and scientific standards.
2. Streamlining Regulatory Compliance
It is difficult to navigate regulatory requirements. AI is useful for:
Automated Documentation:
AI automates the creation and management of regulatory documents, which reduces manual errors.
AI Systems Monitor Processes to Ensure Compliance:
AI systems continuously monitor processes to ensure compliance and alert them to any potential noncompliance.
AI Risk Assessment:
AI can assess the risks associated with processes and take proactive measures to ensure compliance.
Automated Document Translation:
Screenshot from ECI Link Machine Translation
AI can be easily incorporated into your workflow to translate clinical, non-clinical, regulatory, medical device, and CMC (Chemistry, Manufacturing, and Controls) documents. It delivers translations directly in your target language while preserving the original layout and formatting—so there’s no extra work needed to reformat the files.
Automated Medical Writing:

Screenshot from ECI Link Medical Writing
AI applications in the life sciences can also support rapid medical writing. Based on the information you provide (e.g., abstracts), AI can assist in generating drafts and adaping them for different document types.
Automated QE (Quality Evaluation):
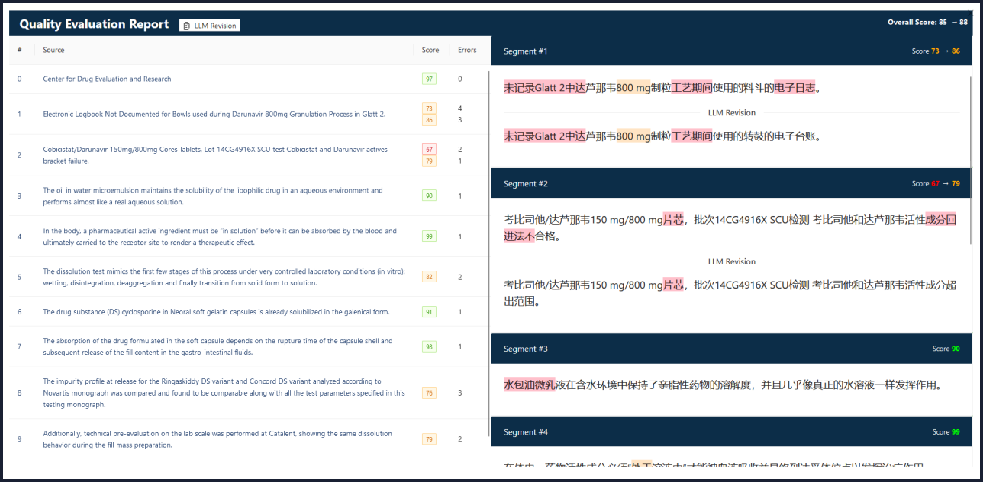
Screenshot from ECI Link Quality Evaluation
After completing automated translation and medical writing tasks, AI can automatically generate a QE report to evaluate content quality. It flags errors based on different scoring criteria. If the score falls below a defined threshold, the content is flagged for human review.
Life sciences companies can use AI to not only maintain compliance but also accelerate complex clinical translations.
3. Enhancing Clinical Trials
Clinical trials are at the core of drug discovery. However, they are often hindered by logistical, regulatory and operational issues. Inefficient patient recruitment, long timelines, and high costs are the main obstacles. Artificial intelligence (AI) is an effective tool to address this problem, as it improves accuracy and speeds up outcomes.
Patient Stratification:
Identification and enrollment of eligible oarticipants is one of the most critical and time-consuming phases of a clinical trial. AI can improve this process by analyzing large volumes of data, ranging from electronic health records and genomic information to electronic health records. Advanced machine learning algorithms can analyze a patient’s disease history, biomarkers, and progression to predict trial eligibility. This leads to a quicker recruitment process, improved patient matching, and a larger representative population.
Trial Design Optimization:
AI can improve trial design by simulating a wide range of scenarios. AI can assist researchers in selecting the best endpoints and dosage strategies, as well as patient cohorts. This is done by analyzing historical trial data and predicting possible outcomes. The predictive model allows for more adaptive and personalized trial frameworks. This reduces the time required for development by minimizing waste.
Real-time Monitoring and Predictive Analytics:
AI-driven tools constantly monitor ongoing trials. These systems are able to detect anomalies such as protocol deviations and unexpected adverse events. Machine learning and natural language processing models examine clinical notes and lab results as well as patient feedback in order to detect data inconsistencies.
This proactive surveillance increases patient safety and ensures compliance with regulations and data integrity throughout the lifecycle of a clinical trial. AI allows us to move away from rigid and outdated models towards dynamic data-driven approaches. These methods can reduce costs, accelerate development, and increase the chances of trial success.
4. Navigating Evolving Regulatory Frameworks
FDA Guidance:
The FDA has released draft guidance on AI/ML and medical devices as well as the use of AI in regulatory decisions. The FDA outlines the principles of model validation, lifecycle, and transparency.
EU AI Act:
The EU AI Act categorizes AI Systems according to their level of risk and mandates some requirements such as data governance and documentation. This is to ensure accountability and traceability.
Global Harmonization:
The FDA, EMA, and PMDA work together on harmonized guidelines, mutual recognition agreements, and streamlined approvals for multinational trials.
5. Addressing Ethical and Privacy Concerns
Data Privacy:
AI solutions must comply with HIPAA, GDPR, and other regulations. To protect patient privacy, they must use techniques like de-identification and secure multiparty computation.
Algorithmic Bias:
To prevent skewed results, AI models are validated and trained on diverse datasets that are representative of the population. Performance metrics are also monitored for different demographic subgroups.
Transparency:
AI frameworks, such as audit trails or model cards, that are easy to comprehend will make AI decision-making more transparent and auditable by regulators and ethics panels.
Conclusion
AI integration into trial design, compliance with regulatory requirements, and ethical oversight are reshaping life sciences. AI enables organizations to navigate global regulatory landscapes, improve patient outcomes and accelerate drug development with greater precision. Although there are still challenges, especially in areas like ethics and data privacy, the continued adoption of AI indicates a more innovative and efficient future for the industry.